Federal Circuit Overturns Seagen’s $42M Patent Win Against Enhertu®

By Bonnie Choi, December 5, 2025
On December 2, 2025, the Federal Circuit reversed the $42M jury verdict against Daiichi’s Enhertu®, ruling that Seagen’s US Patent No. 10,808,039 was invalid for lack of written description and enablement.
Why this matters?
- This decision further reduces the value of broad genus claims in the US.
- Amgen v. Sanofi focused on the enablement requirement for genus claims, indicating that the number of examples needed to support a broad genus needs to be commensurate with the size of the genus. However, Amgen did not address written description.
- As now clarified by Seagen, the written description requirement for a narrow species/subgenus within a broad genus is not satisfied if a patent specification includes only a vague and piecemeal description of the species/subspecies. A patentee must expressly describe a particular species/subgenus when the claims focus on the narrower species/subgenus that falls within a much larger group of possibilities.
- The Seagen decision exemplifies that the Federal Circuit will be taking a similar view on the enablement and written description requirements. For example, the courts will require evidence of inventors actually ‘possessing’ specific species/subgenera that fall within a broad genus. The evidence may be of actual physical possession or actual intellectual possession.
Background and Key Dates:
- Antibody-drug conjugates (ADCs) generally contain three parts: antibody, drug, and linker that connects the antibody and the drug.
- In 2004, Seagen filed a non-provisional patent application on ADCs.
- In 2011, Daiichi invented the ADC Enhertu®. Its structure became public in 2015. Enhertu® contains a tetrapeptide linker made up of only glycine (Gly) and phenylalanine (Phe) residues.
- In 2019 (following the public disclosure of the structure of Enhertu®), Seagen filed a continuation patent application (claiming priority back to the earlier **2004 **application) claiming ADCs where: the linker portion contained a “Ww” tetrapeptide unit made up of only Gly and Phe residues, the drug portion was defined as “a drug moiety [that] is intracellularly cleaved in a patient,” and the antibody portion was any antibody. This application granted as US Patent No. 10,808,039 (the ’039 patent). The scope of the granted claims would generically cover Enhertu®.
- In 2020, shortly after the ’039 patent was granted, Seagen filed suit against Daiichi, alleging Enhertu® infringed the ’039 patent. A jury trial was held in 2022 with the jury finding that Enhertu® did infringe the ’039 patent, and Seagen was awarded ~$42M and an 8% running royalty in damages. Daiichi appealed.
- In parallel with the suit, Daiichi petitioned for a post-grant review of the ’039 patent at the U.S. Patent Trial and Appeals Board (“PTAB”). In 2024, the PTAB held that the ’039 patent was invalid for lack of written description and enablement. Seagen appealed.
Written Description:
- As noted above, the ’039 patent claimed ADCs where the linker portion contained a “Ww” tetrapeptide unit made up of only Gly and Phe residues. Because Phe can be one of two different stereoisomers (in addition to Gly’s one), the claimed subgenus encompassed 81 unique Gly/Phe-only tetrapeptides (based on 3^4). Meanwhile, the specification of the patent described 83 possible residue options beyond just Gly and Phe for any unit within Ww, which corresponded to a genus covering 47 million unique tetrapeptides (based on 83^4).
- The patent specification included examples such as a tetrapeptide Gly-Phe-Leu-Gly, but none that contained only Gly and Phe as claimed.
- Importantly, the inventors of the ’039 patent admitted that they never contemplated ADCs with Gly/Phe-only tetrapeptides in 2004. The first time they ever saw a Gly/Phe-only tetrapeptide in an ADC was Daiichi’s Enhertu® in 2015.
- Seagen argued that In re Driscoll (1977) supported the validity of the claims.* In re Driscoll *concerned US Patent No. 4,429,858 in which the patent claim recited a subgenus of compounds with one specific moiety, whereas the patent specification described 14 specific moieties. However, the court rejected this argument (14 specific moieties in In re Driscoll vs. 47 million possible options here).
- The court found that “although the 81 claimed Gly/Phe-tetrapeptides are ‘encompassed’ within those 47 million…, they are merely an infinitesimal fraction of those peptide units generally included [in the specification].” Thus, the Federal Circuit held that the broad genus disclosure was inadequate to satisfy the written description for the claimed subgenus.
Enablement:
- The ’039 patent claimed ADCs where the drug portion was defined as “a drug moiety [that] is intracellularly cleaved in a patient.”
- The examples in the patent were limited to dolastatin- and auristatin-based ADCs.
- The court cited Amgen v. Sanofi, where the Supreme Court found an entire genus of antibodies that bind to specific amino acid residues on a protein and block that protein from binding to certain receptors was not enabled by the patent, which showed only 26 antibodies out of at least millions of possible antibodies.
- As in Amgen, the court found ADC science to be “so unpredictable” that one of skill in the art would be required to evaluate whether any given ADC with any drug meets that functional limitation of being successfully cleaved in the patient. As the skilled artisan would be left “to perform far-ranging ‘trial-and-error discovery’ to make and use the ADCs within the scope of the ’039 patent,” the court held that the claim lacked enablement.
For reference, the independent claim of the ’039 patent at issue is the following:
- An antibody-drug conjugate having the formula:

or a pharmaceutically acceptable salt thereof, wherein:
Ab is an antibody,
S is sulfur,
each —Ww— unit is a tetrapeptide; wherein each —W— unit is independently an Amino Acid unit having the formula denoted below in the square bracket:
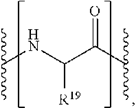
wherein R19 is hydrogen or benzyl,
Y is a Spacer unit,
y is 0, 1 or 2,
D is a drug moiety, and
p ranges from 1 to about 20,
wherein the S is a sulfur atom on a cysteine residue of the antibody, and
wherein the drug moiety is intracellularly cleaved in a patient from the antibody of the antibody-drug conjugate or an intracellular metabolite of the antibody-drug conjugate.